http://pubs.rsc.org/en/Content/ArticleLanding/2012/CC/C2CC33185C?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FCC+%28RSC+-+Chem.+Commun.+latest+articles%29&utm_content=Google+Reader
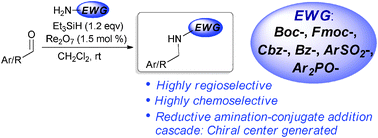
The authors here describe a direct reductive amination procedure using a oxo-rhenium catalyst and silanes as the hydride source. They were able to produce amines at room temperature using 3% catalyst loading without getting over alkylation.

No comments:
Post a Comment