http://pubs.rsc.org/en/content/articlepdf/2012/sc/c2sc20806g
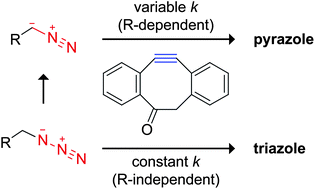
The group reacts cyclooctyne derivatives with diazo compounds and compares their reactivity to the azide counterparts. Polar protic solvents accelerated the reaction, as well as extending conjugation of the diazo group.
Figure above shows how the group synthesized these diazo compounds from azido starting materials. Figure belows show energies and experimental yields for the azido and diazo reaction with a cyclo-octyne derivative.

No comments:
Post a Comment